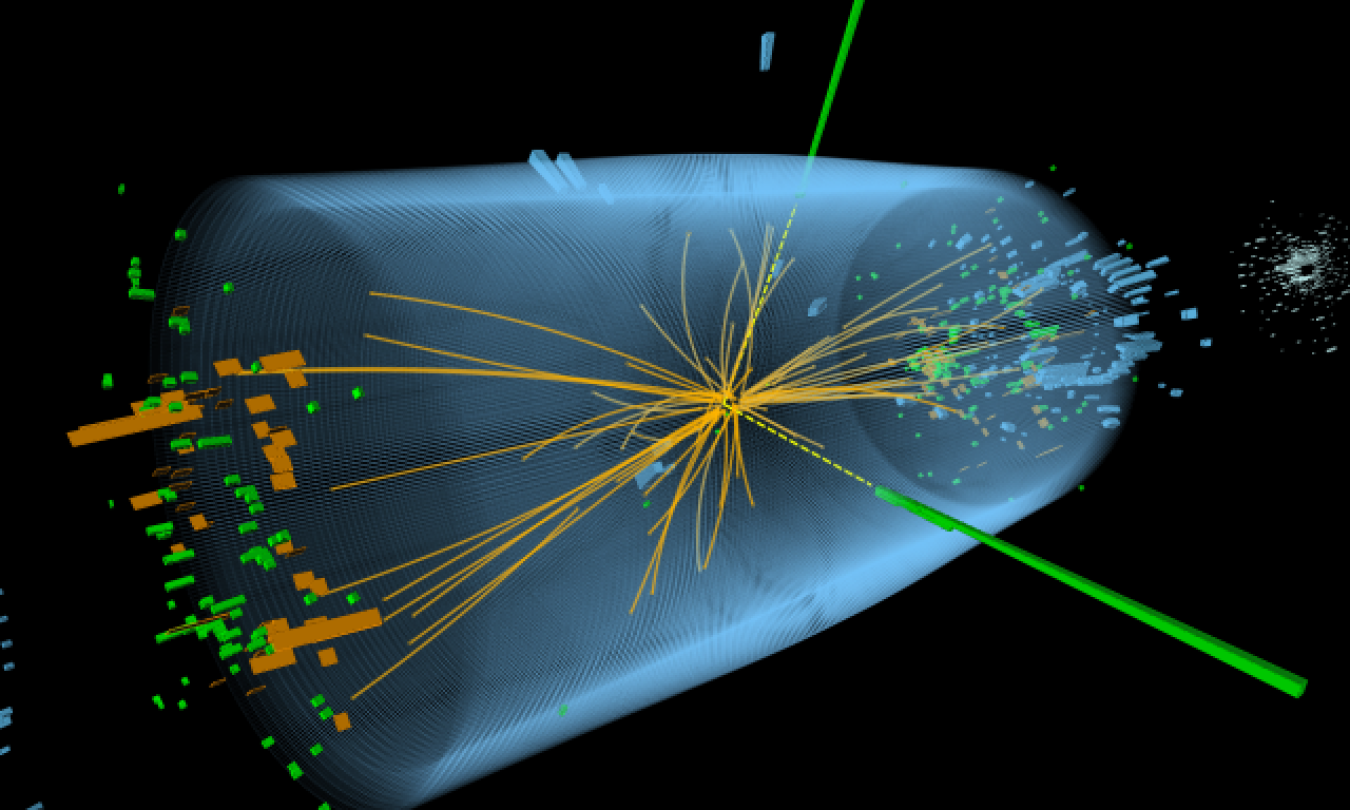
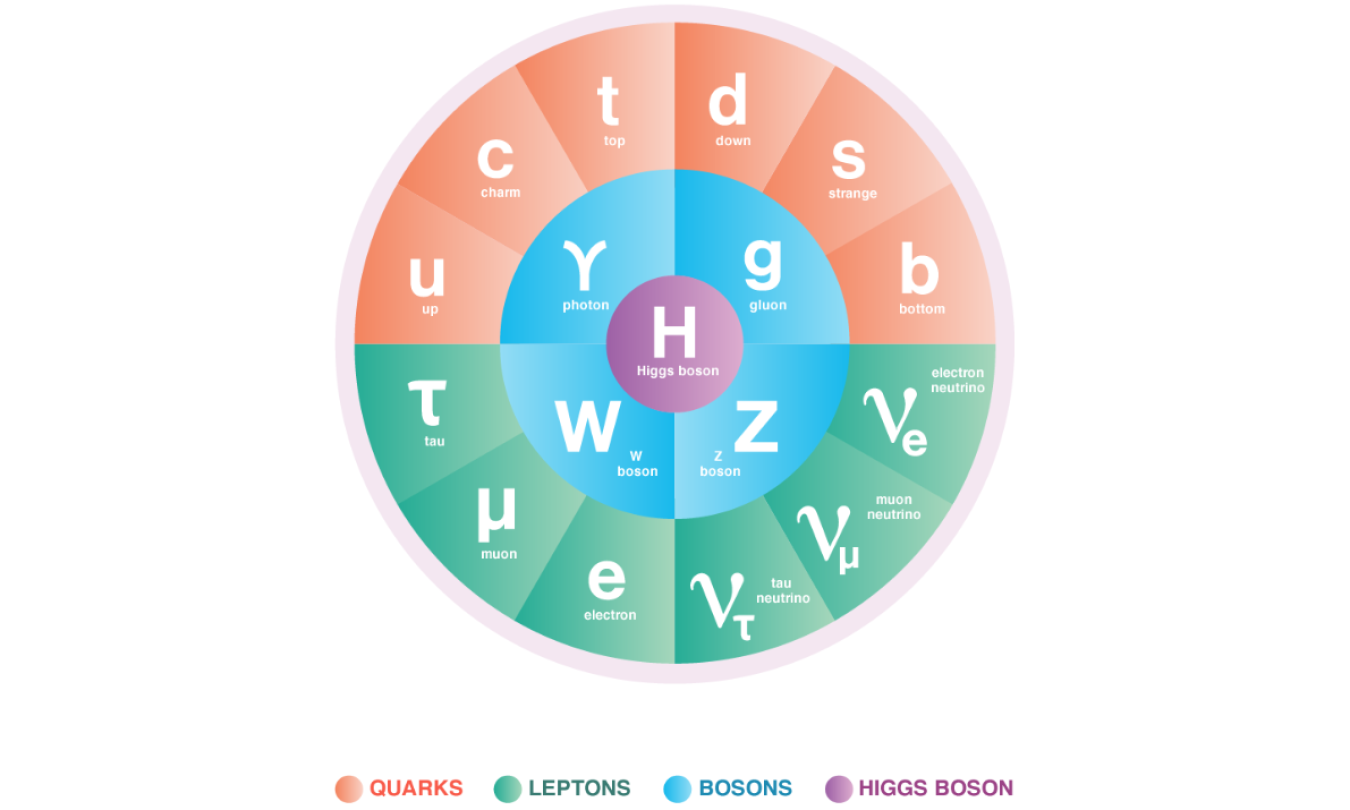
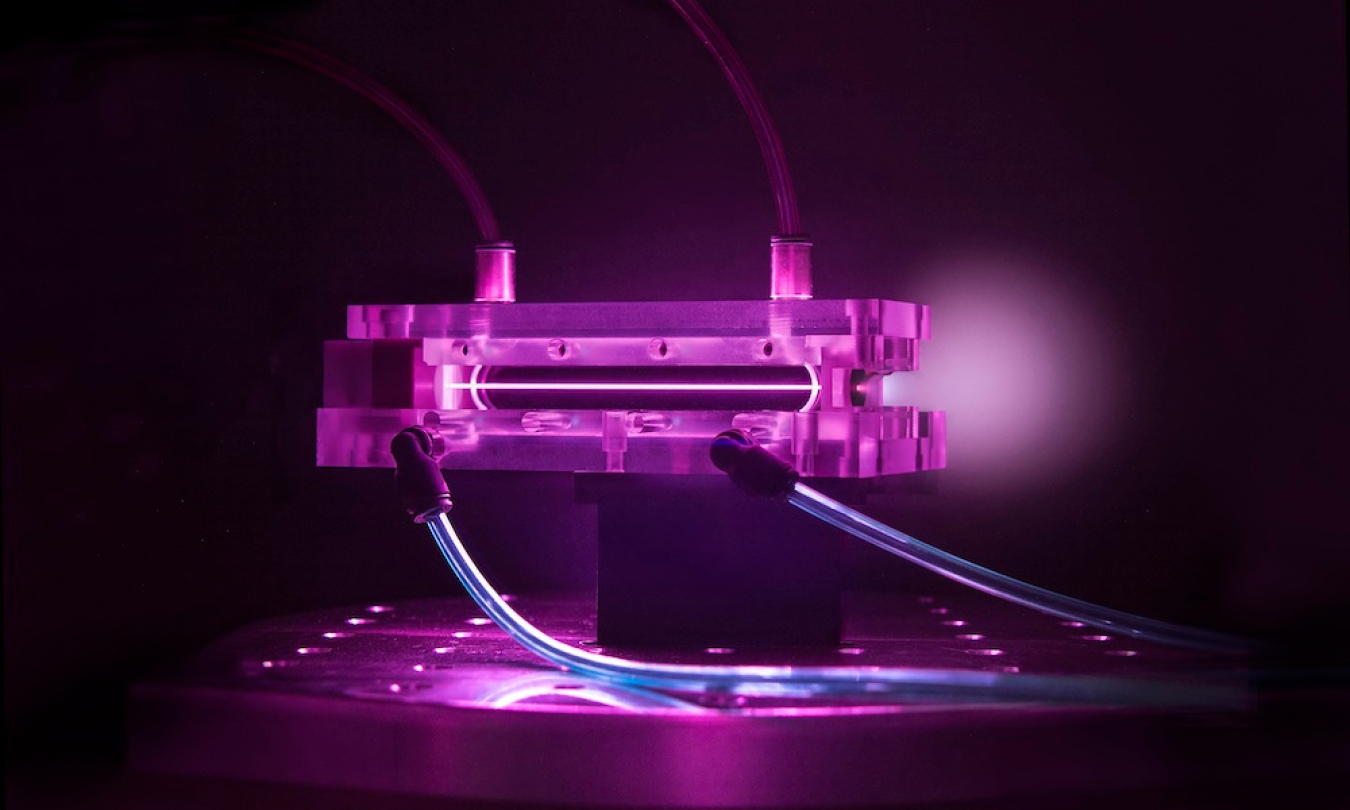
High Energy Physics (HEP) explores what the world is made of and how it works at the smallest and largest scales, seeking new discoveries from the tiniest particles to the outer reaches of space. This quest inspires young minds, trains an expert workforce, and drives innovation that improves the nation’s health, wealth, and security.
Our research is inspired by some of the biggest questions about our universe. What is it made of? What forces govern it? How did become the way it is today? Finding these answers requires the combined efforts some of the largest scientific collaborations in the world, using some of the most sensitive detectors in the world, at some of the largest scientific machines in the world.
We support U.S. researchers that play leading roles in these international efforts and world-leading facilities at our National Laboratories that make this science possible. We also develop new accelerator, detector, and computational tools to open new doors to discovery science, and through Accelerator Stewardship, work to make transformational accelerator technology widely available to science and industry.
Learn more about the High Energy Physics mission and how we support it.
HEP Subprograms
Energy Frontier
Researchers at the Energy Frontier use the world’s largest and highest energy particle accelerator to recreate the universe as it was a billionth of a second after the Big Bang.
Intensity Frontier
Researchers at the Intensity Frontier investigate some of the rarest particle interactions in nature and subtle effects that require large data sets to observe and measure.
Cosmic Frontier
Researchers at the Cosmic Frontier use naturally occurring cosmic particles and phenomena to reveal the nature of dark matter, cosmic acceleration, and more.
Theoretical, Computational, and Interdisciplinary Physics
Theoretical, computational, and interdisciplinary physics provide the vision and the framework for extending our knowledge of particles and the universe.
Advanced Technology R&D
Cutting-edge research in the physics of particle accelerators, particle beams, and particle detection enables scientists to stay on the threshold of discovery.
Accelerator Stewardship
Supporting use-inspired basic research in accelerator science and technology to make particle accelerator technology widely available to science and industry.
HEP Science Highlights
HEP Program News
HEP Research Resources
Contact Information
High Energy Physics
U.S. Department of Energy
SC-25/Germantown Building
1000 Independence Ave., SW
Washington, DC 20585
P: (301) 903-3624
F: (301) 903-2597
E: sc.hep@science.doe.gov