You are made of force fields
 |
| A karate expert can perform feats that most of us can't. A swift kick can break a board. But how does that work on the atomic level?
|
After a quick glance over his shoulder to locate the target and a deft, cat-like pivot on his left foot, the sensei swings his right heel into the boards, breaking them cleanly in half. The students are impressed by the black belt's technical skill. With a single strike, he has shown his impressive mastery of matter.
We all know what matter is. It's you. It's the chair you're sitting in. It's the Earth you're living on and the air you're breathing. But what is it really? You and the chair seem pretty solid; you don't fall through it like a skydiver falls through air. So how does that all work?
As a little kid, you were told that there were three states of matter: solid, liquid and gas. The reason you could move through air was that it wasn't very dense and the molecules could move freely — there was plenty of space between them. In contrast, the molecules in solids were all nestled together. You probably even saw pictures in your textbook like the one on the bottom of the second figure, with all the atoms in solids touching one another and no space between them. These were nice and comforting images. They're also very misleading.
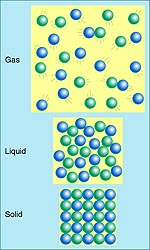 |
| In introductory textbooks, solid matter is shown as a bunch of spherical atoms packed in an orderly way, with no space between them. But just how accurate is that picture?
|
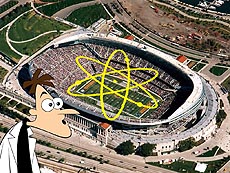 |
| If Dr. Doofenshmirtz (of "Phineas and Ferb" fame) invented an "atomic grow-i-nator" that made a proton the size of a BB, it would generate an atom the size of an NFL football stadium.
|
You've seen pictures of an atom that looks a little like a solar system. (The pedantic reader will note that this picture is obsolete. Hush. For purposes of this article, the model is entirely adequate.) Protons and neutrons inhabit the center of atoms, while electrons swirl around at large distances, in rough analogy to the sun and the planets. Just as there is an awful lot of empty space between the sun and Earth, so it is in an atom.
We can get a sense of just how empty an atom is by taking an ordinary carbon atom and scaling it up. Carbon consists of six protons and six neutrons clumped together, with six electrons orbiting the clump. If you had some sort of "atom growing" device that could enlarge the atom enough so that each proton and neutron was the size of a BB (a few millimeters across), the atom itself would be over a hundred meters across. Indeed, the expanded atom can be envisioned as a marble (the nucleus) sitting at the 50 yard line of a professional football stadium and six grains of sand (the electrons) in big enough orbits to fill the stadium entirely. The atom is almost entirely empty space, with only about one part in a trillion of the atom's volume containing "stuff."
So if matter is mostly empty space, why can't you walk through a wall? It's not because the atoms that make up matter bump into one another. It's because the atoms are held in place by the electromagnetic force. That's right: You and all solid matter are really nothing more than an empty shell filled with force fields. And the reason you slam into a wall is that your electromagnetic force fields run into the force fields of the wall, and the force fields are so strong that you just can't get through.
When the karate expert breaks the board, he has slammed together the board and heel force fields with enough energy to overcome the force holding together the molecules in the wood. But even Chuck Norris couldn't generate enough speed to pass his hand entirely through the board. (Although Chuck Norris did once fight Superman on a bet. The loser had to start wearing underwear on the outside of his pants.)
You've probably known about the basic structure of atoms and ideas I've mentioned here. I'm just pulling them all together to highlight their ramifications. The atomic world is very different from the ordinary world we understand intuitively, with incredible implications for our understanding of the fundamental nature of the universe.
—Don Lincoln
Want a phrase defined? Have a question? E-mail today@fnal.gov.
|